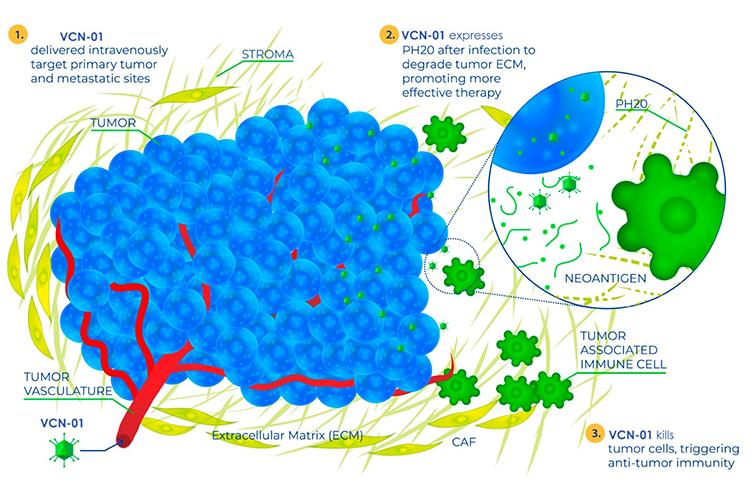
Theriva Biologics, member of CataloniaBio & HealthTech, announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track Designation (FTD) to lead clinical candidate VCN-01 in combination with gemcitabine and nab-paclitaxel to improve progression-free survival and overall survival in patients with metastatic pancreatic adenocarcinoma.
“The FDA’s decision to grant FTD to VCN-01 highlights the urgent need for new treatment options for PDAC, which accounts for the 4th highest cause of cancer-associated deaths in the US and Europe,” said Steven A. Shallcross, Chief Executive Officer of Theriva Biologics. “VIRAGE, our Phase 2b trial evaluating VCN-01 in metastatic PDAC continues to progress, with enrolment expected to complete in the third quarter of 2024. FTD is an important step that furthers our ability to expedite the review of and build upon the compelling clinical data that underscores VCN-01’s multiple modes of action and therapeutic potential in combination with chemotherapy or immunotherapy. We will continue to deliver on our mission to advance new therapeutic options for these patients.”
FTD is designed to help treatments reach patients faster by facilitating the development and expediting the review of therapies with potential to treat serious conditions and fill an unmet medical need. Benefits of FTD to programs include early and frequent interactions with the FDA during the clinical development process and, if relevant criteria are met, the FDA may also review portions of a marketing application before the sponsor submits the complete application.
Comments