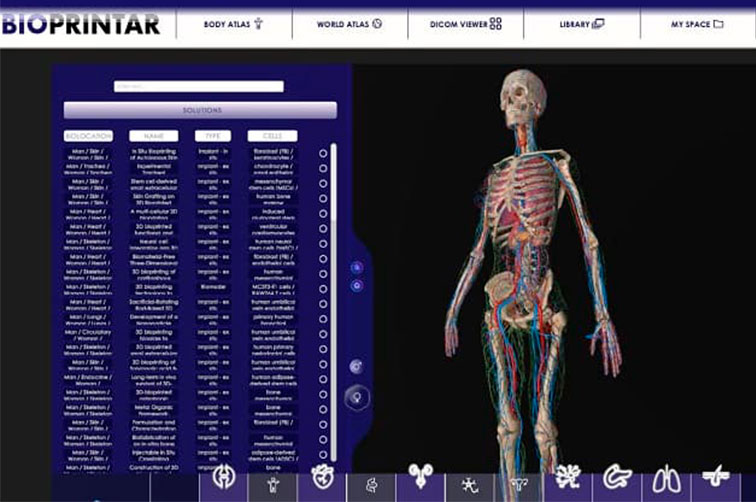
The engineering company Biorem, a member of CATALONIA.HEALTH, has developed a virtual reality platform that allows to visualize bioprinting solutions of organs and tissues on the human body. The company directs the tool to health professionals, doctors, researchers and pharmaceutical product developers so that they can identify real products available worldwide for both clinical trials and bioimplants.
The project has received support from the Government, through ACCIÓ, of about 166,500 euros from the line of Grants for research and development projects. For the development, Biorem has had the collaboration of the GILAB research group of the University of Girona, experts in image processing, 3D visualization and virtual and augmented realities techniques, which has the TECNIO accreditation granted by ACCIÓ.
The company is just developing the platform and working on the business model with the aim of launching it to the market by 2025. At the same time, they are working on developing the version of this system adapted to virtual reality that can be used through glasses. At the same time, they also seek to incorporate new functionalities into the platform that increase their capacity to personalize and automate the results.
Georgina Vidal-Gavilan, director of innovation at BIOREM, explains: "Our team dedicated to innovation, which is very up to date with the new technologies that come out on the market, detected the deployment of 3D technologies in clinical reality. While in the field of medical devices these technologies have been applied for some time, bioprinting is a step beyond that goes from products to models and therapies, an area that involves technical and regulatory difficulties, but also represents an opportunity and that is why we decided to specialize in it”. In addition, she adds: "We specialise in the management of the entire medical technology cycle: from the detection of needs, to planning, acquisition and deployment, to the maintenance of this technology, with a special focus on complexity management in order to optimize the application of equipment and technology in health and pharmaceutical environments."
Comments